New Mexico Heart Institute
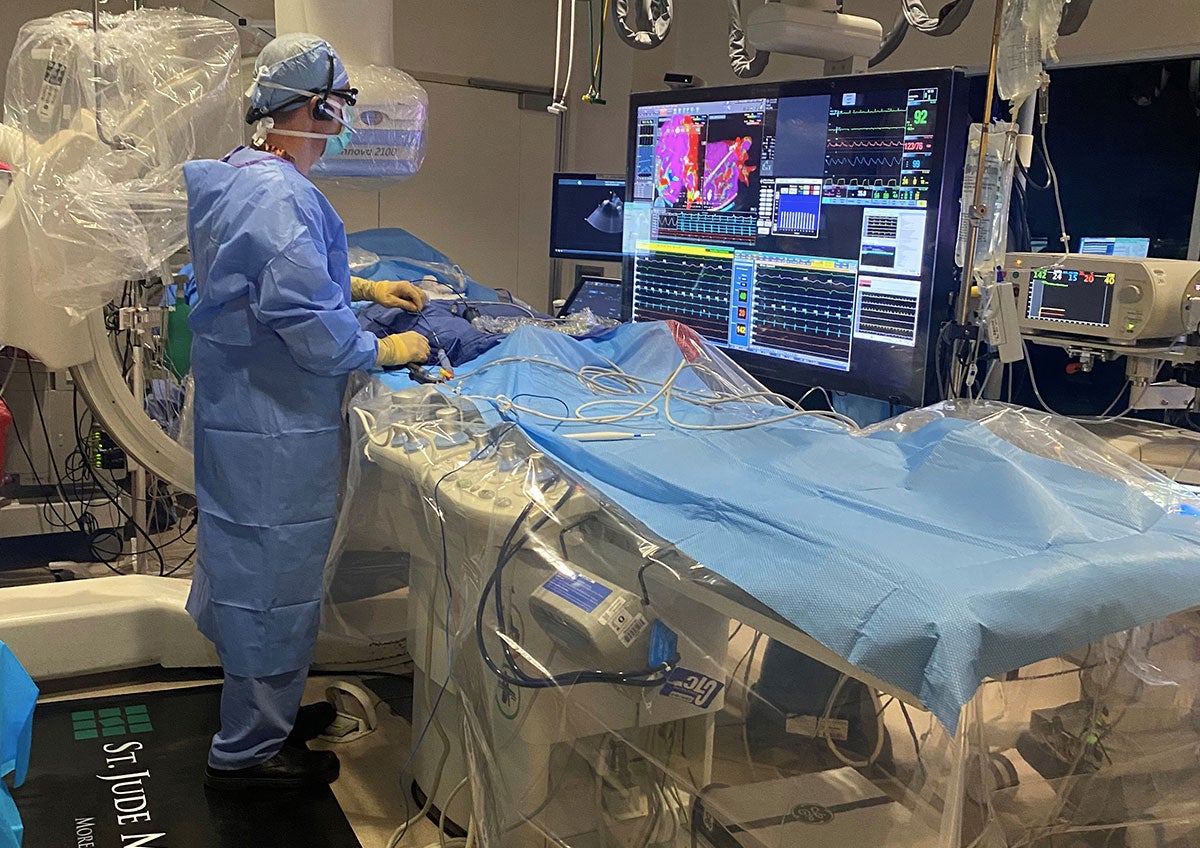
The New Mexico Heart Institute (NMHI), a department of Lovelace Medical Center, is a leader in the nation when it comes to research and clinical trials as it relates to cardiac care. Our cardiology team is committed to finding treatments for a variety of cardiac conditions. Clinical Research also plays a vital role in the development of products to combat diseases, treat chronic and degenerative diseases and improve the health of people around the world.
Our Mission
The Clinical Research Department at Lovelace Medical Center is an innovative team dedicated to engaging in pioneered science for global healthcare solutions to enhance the health of communities we serve.
Our team of skilled experts work directly with our patients to help educate about the trial, gather necessary data, treat the patients accordingly, and help manage our patients care.
Meet our Researchers
Meet the Team

Keisha Eggins, CCRP
Clinical Research Manager

Rebecca Jones, BSN, RN
Clinical Research Nurse

LaTasha Johnson
Clinical Research Coordinator

Selena Abeita
Clinical Research Coordinator

Alexandra Ortiz, CCRC
Clinical Research Coordinator

Zinai Tellez, CCMA
Clinical Research Coordinator
Types of Clinical Trials Available for Participants at NMHI
Our reasearch team and providers are involved in multiple trials for the treatment of heart and vascular diseases. Each research trial is carefully monitored by an independent review board to protect the rights and welfare of the participants. Below are the types of cardiovascular diseases NMHI is currently conducting clinical research and trials for:
FAQ
















